Inside BioNTech - The Facilities Making the World's Most Prevalent COVID-19 Vaccine
© Luca LocatelliIf you’ve been vaccinated for COVID-19, chances are pretty high that you’re benefiting from a product made by BioNTech. The German biotech company, co-founded by a husband-and-wife team of scientists, developed the vaccine that became not only the first to earn authorization in the U.S. for COVID-19 in December but also the first ever based on a new technology involving the genetic material mRNA.
In interviews in December and March, co-founders Ugur Sahin and Ozlem Tureci spoke about their whirlwind year and their partnership with U.S. pharmaceutical company Pfizer to test and manufacture the vaccine. Over three days in late March, they also opened up their new manufacturing plant to TIME for the first step-by-step look at how their lifesaving, and potentially pandemic-ending, vaccine is made.
When Sahin read a scientific paper in late January 2020 describing the first identified cases of COVID-19 in Wuhan, China, “it was very clear to me that this was not a local outbreak anymore,” he says. “And most likely the virus had already spread worldwide.”
He knew there was no time to waste. But BioNTech, based in Mainz, was then primarily a cancer-vaccine company; after more than a decade of research and development, the company had tested its mRNA-based cancer vaccines in about 400 people, with encouraging results. They were just exploring the possibility of creating vaccines against infectious diseases–specifically an mRNA-based vaccine against flu–when COVID-19 hit.
With so little known about the new virus, the team turned to what was known about two related coronaviruses: SARS and MERS. Soon it had a 50,000-step process for building an mRNA vaccine against the new coronavirus, SARS-CoV-2.


It begins in a 50-L stainless-steel tank that more closely resembles a beer keg than what you might imagine to be part of a lifesaving bioreactor. Inside are fragments of mRNA coding for the SARS-CoV-2 spike protein, the Achilles’ heel of the virus that the vaccine will exploit. The entire production process happens in a hermetically sealed system, with products from each stage transported to the next via a network of transparent plastic tubing.
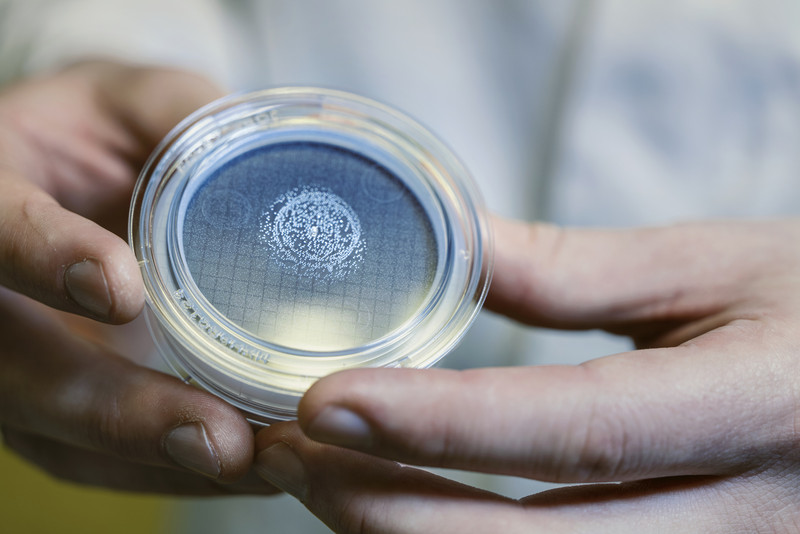
Even so, just to be safe, technicians routinely test the air in the manufacturing rooms for any extraneous bacteria or pathogens, and those working with the vaccine regularly tap each gloved finger onto petri dishes filled with agar that can culture for any stray microbes that might have made their way into the facility.
Because mRNA is relatively unstable, it wouldn’t survive in its raw form in the human body. In order to keep the mRNA protected, it’s encased in a fatty bubble using pressurized ethanol–a highly flammable substance. The process happens in one of six 50-L tanks, each in its own sealed-off room. The few people authorized to venture in and out of the rooms put on special static-free shoes to avoid generating an accidental friction spark that could set off an explosion. Five of the tanks are named for team members who were instrumental in developing the process, and the sixth is named Margaret, after the U.K. grandmother who was the first person to receive the vaccine.
Once the ethanol has done its job of creating the mRNA-containing bubbles, it is filtered out. The result is then filtered several times more, eventually ending up as a milky solution that fills 10-L plastic bags. That liquid is shunted to the so-called fill-and-finish phase, where it is purified in tanks and then squirted into sterile vials–each containing up to six doses–that are shipped to clinics around the world.











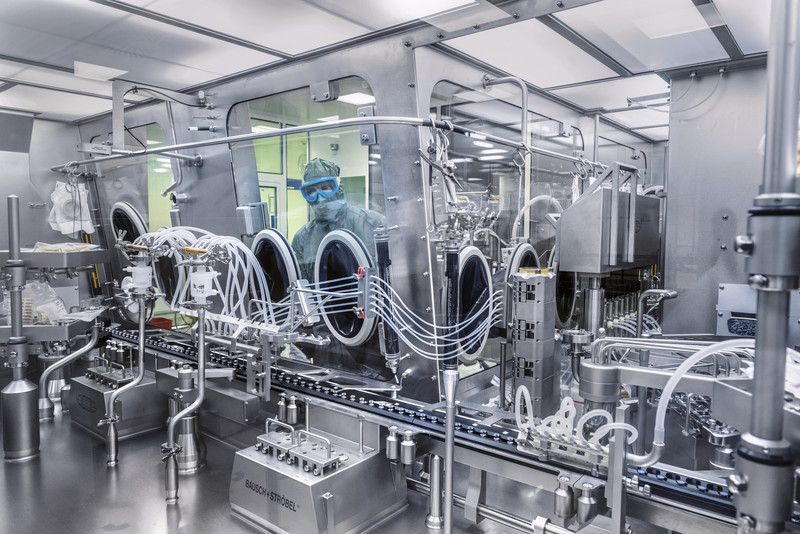

BioNTech currently produces 8 million doses of its vaccine every three to seven days at the new Marburg facility that the company purchased from Novartis last fall. (Many lab technicians, most of whom transferred to BioNTech, still wear their old lab coats with the Novartis logo, as there hasn’t been time to order new ones.) Ultimately, the plant will churn out 1 billion doses a year, and BioNTech is working with Pfizer, which oversaw the final human testing that resulted in the vaccine’s authorization in the U.K., the U.S. and elsewhere, to ramp up manufacturing to produce the 2.5 billion doses it committed to providing the world in 2021.
Both companies are quick to point out that their work is not done. Sahin and his team are also keeping an eye on new variants of the virus starting to take hold around the world, and have already developed another vaccine to target those viral versions, and they plan to start testing it soon.
Text excerpt by ALICE PARK & ARYN BAKER for TIME
click to view the complete set of images in the archive
This story was commissioned by TIME Magazine and was published in the April 26, 2021 issue
